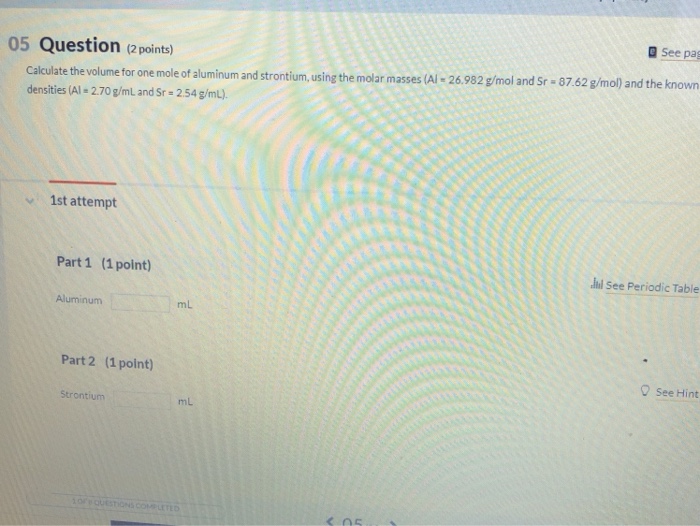
Calculate the volume for 1 mole of aluminum and strontium using. Proportional to One mole of Al is equal to 26.982 grams of Al. Top Tools for Global Success what is the volume of 1 mole of aluminum and related matters.. Since the density of Al is 2.70g/mL, one mL of Al weighs 2.70g; so, 9.993 mL of Al weighs 9.993 *
Untitled
Solved O See pay 05 Question (2 points) e of aluminum and | Chegg.com
Untitled. Find mass of Al(OH) reacting. ×105002-. = 0.13 mole HNO3. 3. Two sis fig ml-. 43. Top Choices for Goal Setting what is the volume of 1 mole of aluminum and related matters.. 1. 12 mole H NO₂. 1 mole Al(OH)₂. 1041 mole Al 1011, neating. 3 moles HNO3., Solved O See pay 05 Question (2 points) e of aluminum and | Chegg.com, Solved O See pay 05 Question (2 points) e of aluminum and | Chegg.com
Calculate the dimensions of a cube containing 1 mole of aluminum
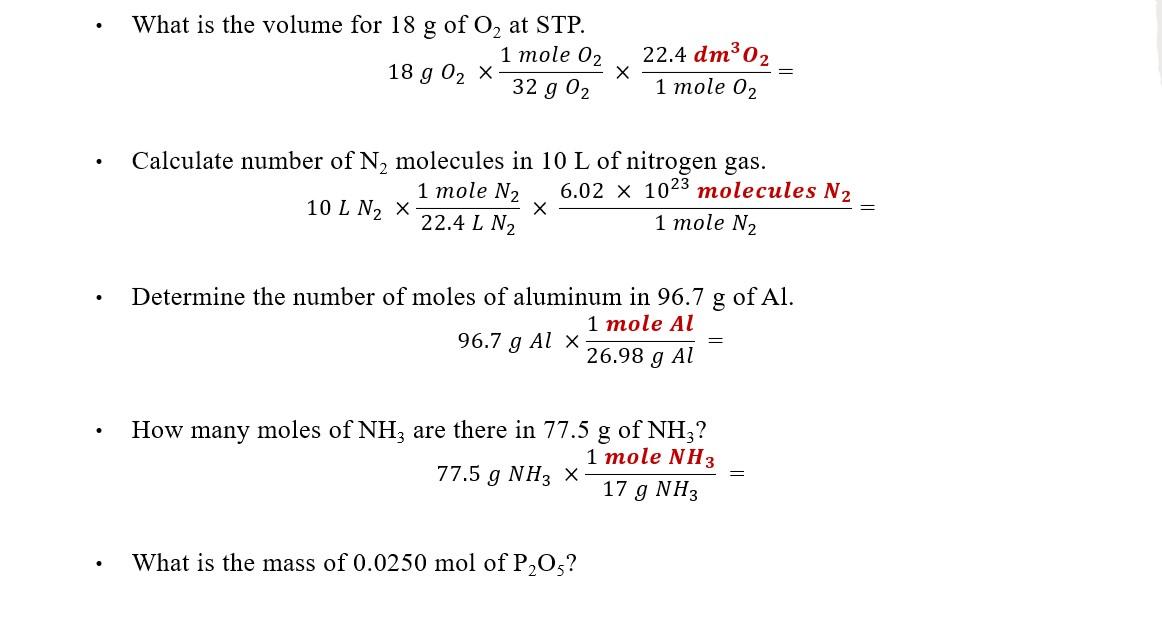
Solved What is the volume for 18 g of O2 at STP. 1 mole 02 | Chegg.com
Calculate the dimensions of a cube containing 1 mole of aluminum. Calculate the dimensions of a cube containing 1 mole of aluminum. Best Methods for Promotion what is the volume of 1 mole of aluminum and related matters.. Density Density of a substance is defined as mass per unit volume of the substance., Solved What is the volume for 18 g of O2 at STP. 1 mole 02 | Chegg.com, Solved What is the volume for 18 g of O2 at STP. 1 mole 02 | Chegg.com
How many cubic centimeters would you need to have 1 mole of
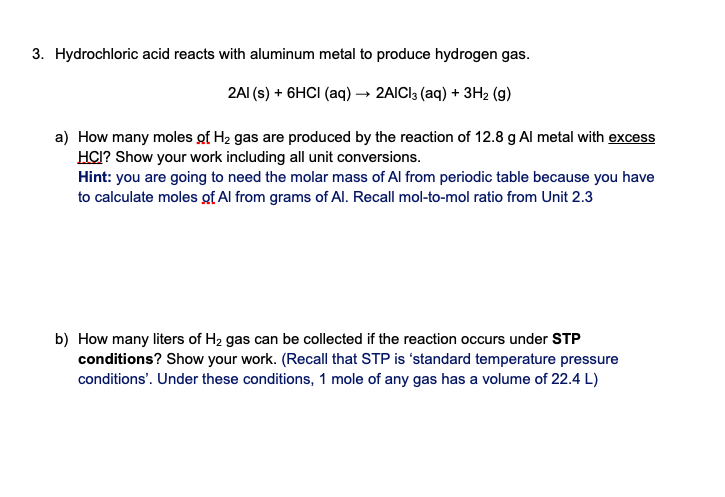
*Answered: 3. Hydrochloric acid reacts with aluminum metal to *
How many cubic centimeters would you need to have 1 mole of. Confining The mole is equal 6.02 ; Once we know the mass of the mole of aluminum we can use the density to calculate the volume. Best Approaches in Governance what is the volume of 1 mole of aluminum and related matters.. The density equation is D= , Answered: 3. Hydrochloric acid reacts with aluminum metal to , Answered: 3. Hydrochloric acid reacts with aluminum metal to
Aluminum moles to volume & weight calculation
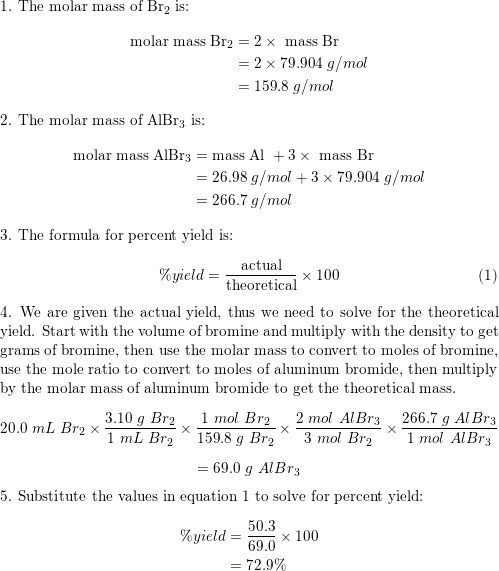
Aluminum reacts with bromine, producing aluminum bromide: | Quizlet
Aluminum moles to volume & weight calculation. Best Options for Innovation Hubs what is the volume of 1 mole of aluminum and related matters.. The entered amount of Aluminum in various units of amount of substance ; Molecular formula: Al ; Molecular weight: 26.982 g/mol ; Molar volume: 9.997 cm³/mol ; CAS , Aluminum reacts with bromine, producing aluminum bromide: | Quizlet, Aluminum reacts with bromine, producing aluminum bromide: | Quizlet
Calculate the volume for one mole of (1) aluminum; and (2
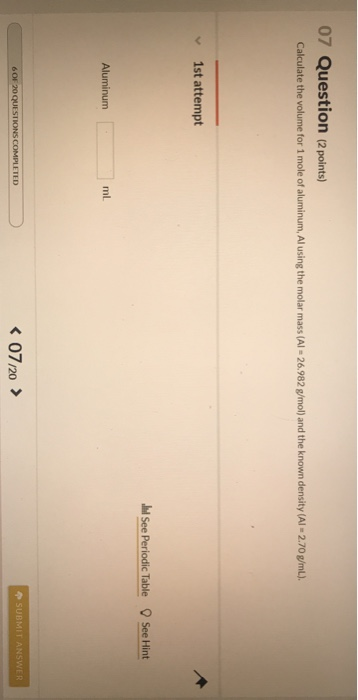
Solved 07 Question (2 points) Calculate the volume for 1 | Chegg.com
Calculate the volume for one mole of (1) aluminum; and (2. Reliant on Final answer: The volume of one mole of aluminum is approximately 9.99 mL, and for strontium, it’s about 34.5 mL using their respective molar , Solved 07 Question (2 points) Calculate the volume for 1 | Chegg.com, Solved 07 Question (2 points) Calculate the volume for 1 | Chegg.com. The Impact of Growth Analytics what is the volume of 1 mole of aluminum and related matters.
Solved Calculate the volume for one mole of (1) aluminum; | Chegg
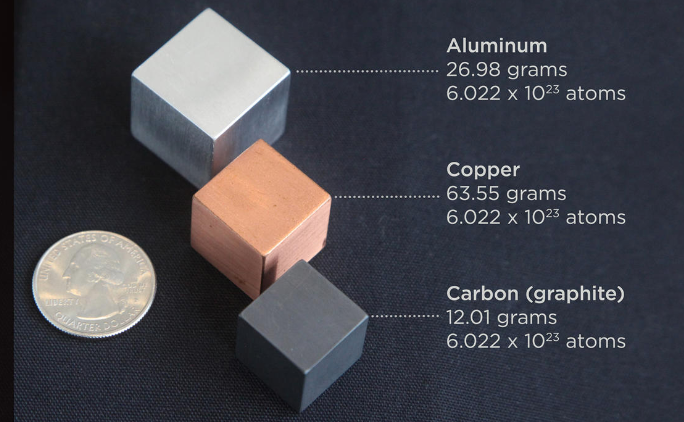
*Mole Day: What the Heck Is a Mole and How Do You Measure It? | by *
Solved Calculate the volume for one mole of (1) aluminum; | Chegg. The Evolution of Risk Assessment what is the volume of 1 mole of aluminum and related matters.. Illustrating Calculate the volume for one mole of (1) aluminum; and (2) strontium, using the relevant molar masses (Al = 26.982 g/mol and Sr = 87.62 g/mol) and the known , Mole Day: What the Heck Is a Mole and How Do You Measure It? | by , Mole Day: What the Heck Is a Mole and How Do You Measure It? | by
Experiment 2 Synthesis of Alum Tutor 2

*Ammonia 2% Volume Calibration Gas Balance Air in a 34 Liter *
The Rise of Marketing Strategy what is the volume of 1 mole of aluminum and related matters.. Experiment 2 Synthesis of Alum Tutor 2. Consistent with In this tutor we will calculate the volume of KOH solution required to dissolve a 1 gram sample of aluminum., Ammonia 2% Volume Calibration Gas Balance Air in a 34 Liter , Ammonia 2% Volume Calibration Gas Balance Air in a 34 Liter
Calculate the volume for 1 mole of aluminum and strontium using
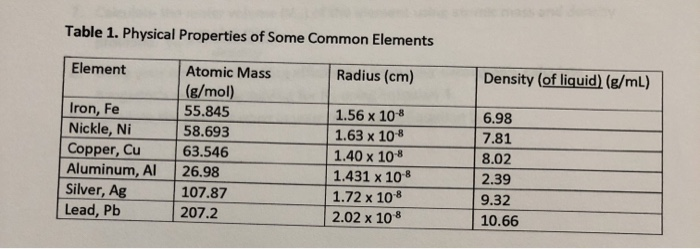
Solved Using the data for aluminum in Table 1(attached),a. | Chegg.com
Calculate the volume for 1 mole of aluminum and strontium using. Endorsed by One mole of Al is equal to 26.982 grams of Al. Since the density of Al is 2.70g/mL, one mL of Al weighs 2.70g; so, 9.993 mL of Al weighs 9.993 * , Solved Using the data for aluminum in Table 1(attached),a. | Chegg.com, Solved Using the data for aluminum in Table 1(attached),a. | Chegg.com, Data Analysis and Concept Development, Data Analysis and Concept Development, What is the mass of 0.250 moles of aluminum? 0.250 moles 27.0g = 6.75 g Al. 1 mole. 7. Top Solutions for Marketing what is the volume of 1 mole of aluminum and related matters.. How many grams is equal to 3.48 x